NEWS
Press release: invIOs reports promising Phase 1b results for cell therapy APN401 in advanced solid tumors
Press release: invIOs to present progress across oncology pipeline during JP Morgan Week 2025
Press Release: invIOs raises €8.2 million in a Series A to finance pipeline progress in immuno-oncology
Funding secures ongoing pipeline progress and achievement of preclinical and clinical milestones Oral small molecule INV501: collaboration with Dana-Farber Cancer Institute (DFCI) making good progress; target validation and details of…
Press release: We are proud to announce that invIOs has selected INV501’s lead candidate to progress into IND-enabling studies in glioblastoma and melanoma.
Three questions to Prof. Gottfried Baier
Press release: invIOs and Dana-Farber Cancer Institute start collaboration to study novel small molecule INV501 for treatment of glioblastoma
• Preclinical study will be conducted by Dr. David Reardon’s research group • Immune-activating small molecule INV501 will be tested as treatment for aggressive brain cancer Vienna, Austria and Boston,…
Press release: invIOs starts further clinical trial of APN401, novel cell therapy against solid cancers, and secures major Austrian grant funding
Multi-center trial of novel autologous cell therapy APN401 leveraging two GMP-compliant manufacturing sites has received regulatory approval and patient recruitment has started; invIOs awarded up to 45% funding of trial…
invIOs’s EPiC cell therapy platform to progress development of novel CAR-T cell approach against lung cancer with Medical University of Innsbruck
Press release: invIOs presents exciting preclinical data showing that immune-activating small molecule INV501 induces strong cytotoxic activity against solid tumors
Lessons from the clinic: Checkpoint blockade, biomarkers, and the future of individualized solid tumor treatments
Press release: invIOs presents promising new clinical data in solid tumors from autologous cell therapy APN401 at AACR Annual Meeting 2023
Meet invIOs’s new Scientific Advisory Board
Clinical trials: From bench to bedside to improve outcomes for cancer patients
Press release: invIOs to present at Biotech Showcase
Three questions to Sarah Bischof, Head of Clinical Operations
Press release: invIOs presents positive patient data from ongoing Phase 1b trial of APN401 cell therapy in advanced solid tumors at SITC 2022
Press release: APEIRON Respiratory Therapies announces positive results from Phase I trial of inhaled APN01
Administration of aerosolized APN01 shown to be safe and well tolerated at all dose levels, with no adverse events above Grade 2 Investigators recommend further development of inhaled APN01, which…
Press release: invIOs to present data from clinical stage Cbl-b program at SITC 2022
Vienna, Austria, 18 October 2022: invIOs GmbH (“invIOs”), a privately held biotechnology company developing novel therapies for cancer, today announces that two abstracts on its clinical stage lead program, APN401,…
Press release: invIOs to present at upcoming industry and investor conferences
Cancer – the hallmarks and beyond
Three questions to Mario Kuttke, Head of Cell Therapy
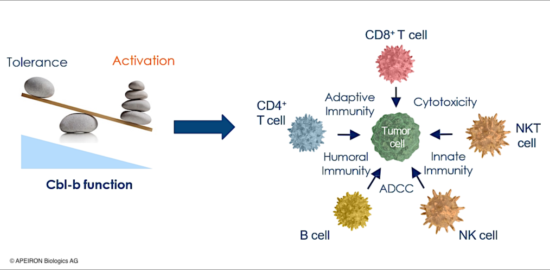
The race against cancer – targeting Cbl-b in cellular therapy
Press release: APEIRON shareholders approve new group structure
Three questions to Alexander Dohnal, Head of Preclinical Development
RNA – in the midst of a medical revolution
Ribonucleic acid (RNA) therapies are promising to change the way we treat diseases. From cancer therapies to vaccines and treatments for genetic diseases, the opportunities are almost infinite.
Press release: invIOs to present at upcoming conferences
Unlocking the master checkpoint of cancer immunity
Our knowledge of cancer is growing daily, bringing with it new treatment strategies. As mentioned previously (here) novel immunotherapies are at the forefront of cancer research. The immune activating monoclonal…
Three questions to Romana Gugenberger, Chief Medical and Scientific Officer
1. Despite novel immune-therapies against cancer there are still non-responders and relapses. Why is that? Romana Gugenberger: Tumors are heterogeneous. Additionally, tumors are not static but evolve. Once a response…
Press release: invIOs goes live
invIOs goes live with unique cell therapy platform and clinical-stage immuno-oncology pipeline Vienna, Austria, 16 December 2021: invIOs (innovative Immuno-Oncology) GmbH, a privately held biotechnology company developing novel therapies for…
The immune system’s fight against cancer
Cancer is the second leading cause of death worldwide. Obviously, the complex cancer biology and simultaneous failure of our body to halt the transformation of a normal cell into cancer…
Perspectives: The Cancer Revolution
Despite recent advances in the treatment of cancer, the disease remains a leading cause of death by disease worldwide1. It is reported that one in two people will be diagnosed…
Press release: APEIRON Biologics launches next clinical trial with innovative cancer therapy APN401
Important development step for promising cell therapy Paradigm shift in cancer treatment: process developed by APEIRON uses immune system to treat solid tumors Innovative new manufacturing and treatment process enables…
Press release: APEIRON Biologics announces changes to management and supervisory boards
Management Board to be expanded by Romana Gugenberger, PhD, as Chief Medical & Scientific Officer (CMSO) and Andreas Gerber, PhD, as Chief Operating Officer (COO) Prof. Josef Penninger moves from…