OUR APPROACHES TO DEVELOPING NOVEL CANCER THERAPIES

- Tumor-specific immune activation and tumor cell killing
- First candidate at pre-clinical proof of concept
- Has potential to establish immunological memory
NEXT-GENERATION CELL-THERAPY PLATFORM (EPiC)
- For treating various solid tumors
- First program generating promising clinical data
- Rapid local cell processing enables out-patient treatment
SCREENING FOR HIGH-POTENTIAL
IMMUNE-ACTIVATING COMPOUNDS
Our latest project focuses on identifying SMALL-MOLECULE APPROACHES with high potential to activate T cells in an antigen-specific manner.
The first candidate compound from this program, INV501, has profound capacity for tumor-specific immune activation. A patient-friendly ORALLY AVAILABLE COMPOUND, it has generated strong data in pre-clinical in vivo testing in multiple solid tumor indications.
For more information on INV501, please see our pipeline.
INNOVATIVE CELL THERAPY PLATFORM
EPiC, the Enhancement Platform for immune Cells, is our platform technology for creating INDIVIDUALIZED CELL-THERAPY TREATMENTS that work with the patient’s own immune system to fight solid tumors.
Based on rapid, localized cell processing in a closed manufacturing system, our platform technology ENABLES OUT-PATIENT TREATMENT for even hard-to-treat solid tumors.
For patients, this means an individualized cell therapy, shorter treatment times, and FEWER SIDE EFFECTS. For clinicians, our system can be easily integrated into daily clinical workflows. And because everything is done locally, we have NO COMPLEX LOGISTICS – the system is easily deployable and scalable.
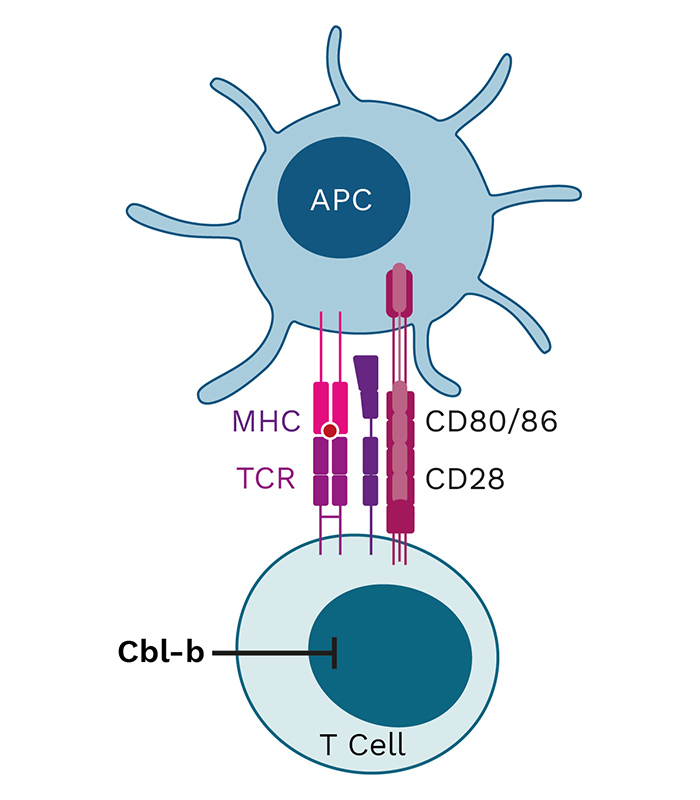
The FIRST PROGRAMS from the EPiC platform, INV441 and APN401, both target Cbl-b, a master immune checkpoint and a negative regulator of T-cell activation. You can learn more about the science of master immune checkpoints and Cbl-b as a target on our blog.
For more information on INV441 and APN401, please see our pipeline.
siRNA-BASED CELL THERAPIES TARGETING CBL-B
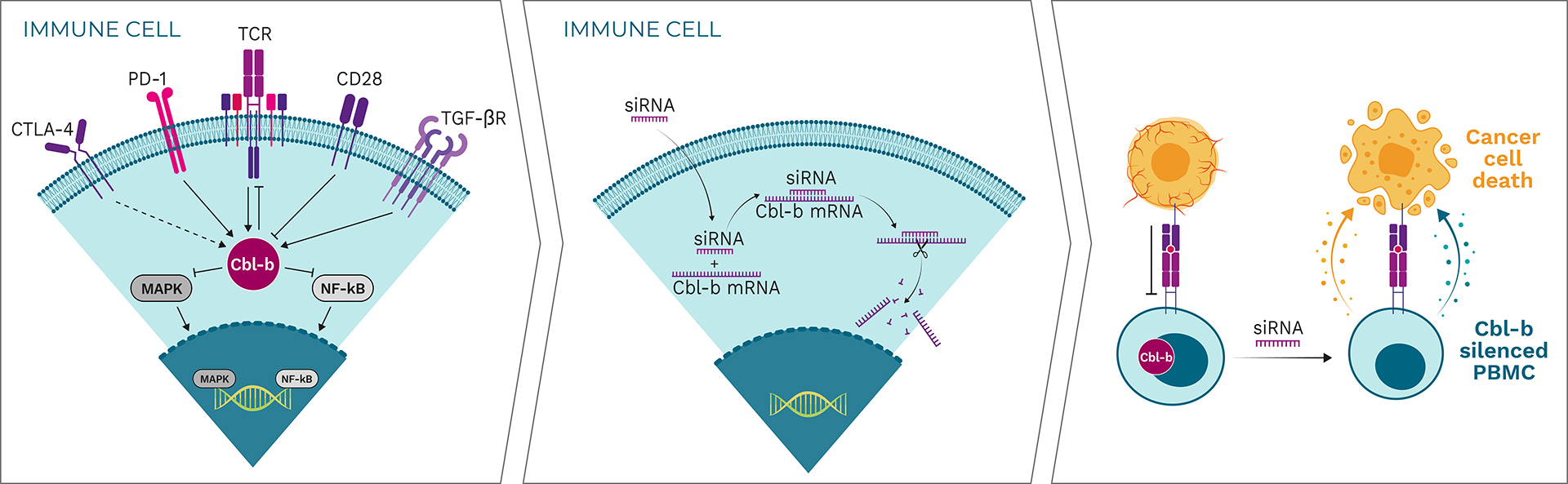
Cbl-b is a negative regulator and a major signaling hub for the activation of tumor-specific immune cells
By “patching” siRNA to the Cbl-b mRNA, Cbl-b is silenced
Cbl-b deficiency activates a variety of immune cells for tumor destruction
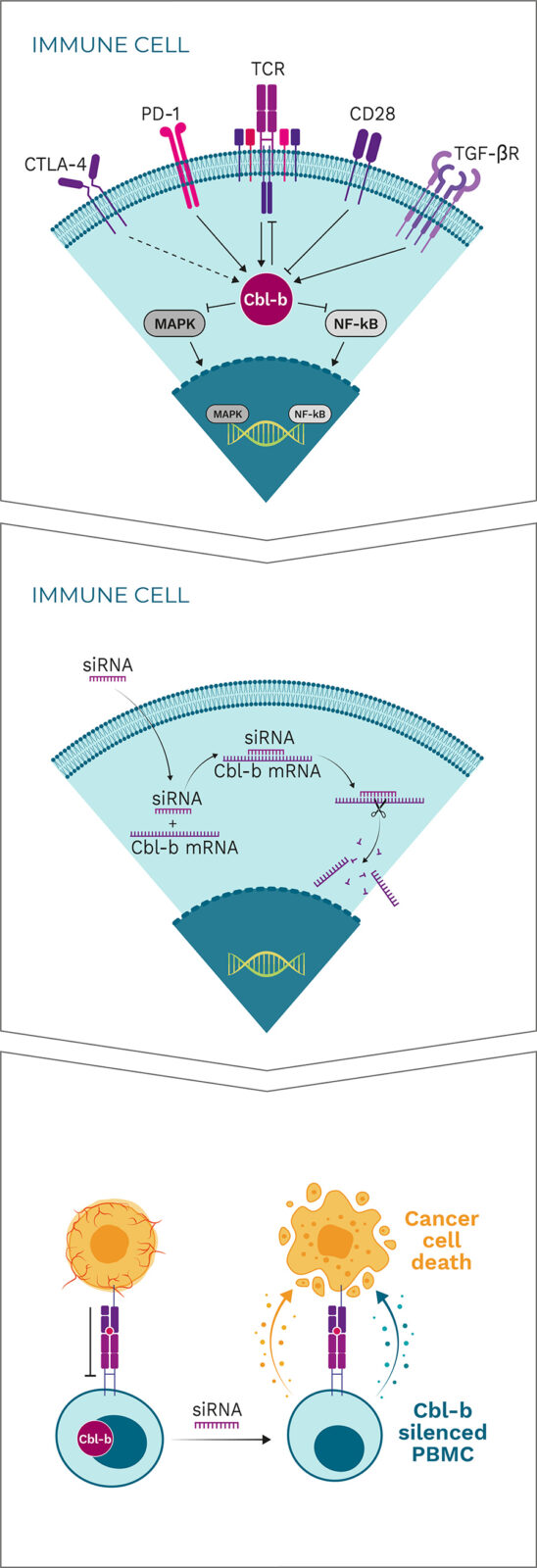
Cbl-b is a negative regulator and a major signaling hub for the activation of tumor-specific immune cells
By “patching” siRNA to the Cbl-b mRNA, Cbl-b is silenced
Cbl-b deficiency activates a variety of immune cells for tumor destruction
Cbl-b is a negative regulator of immune cell activation and has been described as a “master checkpoint” in immune function. Disrupting Cbl-b expression by silencing the Cbl-b gene enhances T cell and NK cell activity and results in reduced tumor growth in animal models. Blocking the Cbl-b function thereby activates both adaptive (T cells) and innate (NK cells) anti-tumor mechanisms, leading to more effective tumor destruction.
APN401 and INV441 are autologous cell therapies designed to inhibit the immune checkpoint Cbl-b using RNAi technology to enhance the immune response in cancer patients. Additionally, they benefit from repeated dosing of freshly modified cells, reducing immune escape of cancer cells.
Cbl-b silencing leads to:
- Proliferation of T cells
- Anti-tumor adoptive immune responses
- Production of cytokines such as IL-2
- Central memory effects
- Reduction in T cell exhaustion
- Lower activation threshold
Cbl-b-silenced PBMCs show a clear increase in proliferation and production of certain cytokines such as interferon gamma (IFN-γ) and interleukin 2 (IL-2) in response to stimulation. Importantly, neither proliferation nor cytokine production are induced in unstimulated T cells, indicating that Cbl-b silencing enhances T cell responses only in the context of antigen stimulation. This approach is superior to generalized systemic activation of all lymphocytes or to systemic administration of cytokines, which are often associated with severe toxicity and may also have negative immunoregulatory effects. Specifically, Cbl-b deficiency enhances anti-tumor activity of T cells and NK cells in vitro, resulting in: enhanced expression of inflammatory cytokines and activation markers when stimulated via the T cell receptor (TCR); enhanced proliferation and anti-tumor cell cytotoxicity; resistance to transforming growth factor beta (TGF-β)-mediated immune suppression (T cells); and increased activation of NK cells upon cytokine stimulation or tumor cell contact.
OUR INNOVATION HUB
DEDICATED TO DELIVERING LIFESAVING IMMUNO-ONCOLOGY TREATMENTS
invIOs has deep expertise and a PROVEN TRACK RECORD of successful preclinical/clinical development of cancer treatments and in-licensing of novel projects.
We want to EXPAND our immuno-oncology (IO) pipeline by leveraging our expertise to DEVELOP product candidates to clinical proof of concept and beyond.
WE AIM TO ACCELERATE PROJECTS USING OUR
WELL-ESTABLISHED ENTREPRENEURIAL INFRASTRUCTURE
Highly experienced staff
Fully equipped lab
Collaboration network
Clinical operations department
CMC unit
Regulatory experts
Patent management
Back-office support
Finance
To complement our own scientific programs, we are looking for:
-
- Innovative, first-in-class targets/projects for IO therapies
- Availability of proof-of-principle data (in vitro or in vivo)
- All oncology indications, including orphan diseases
Submit your request or project offer: innovation@invios.com

