In light of the 21st annual #WorldCancerDay we would like to draw your attention to our work in fighting cancer. We at APEIRON Biologics AG want to make a contribution to people’s #wellbeing by developing the immune therapy APN401. APN401´s proprietary process brings in a paradigm change in cancer treatment to fight hematological and solid tumors.
Reactivated immune cells of cancer patients (APN401) for the treatment of solid tumors
What is APN401 ?
- APN401 is a first-in-class individualized cellular therapy, which is manufactured from patient blood cells followed by immediate re-infusion in an ambulatory setting.
- APN401 blocks the intracellular master immune checkpoint, Cbl-b (Casitas B-lineage lymphoma b), in order to strengthen tumor-specific immune reactivity and killing of tumor cells.
Which benefits does APN401 offer for cancer patients ?
- APN401 follows an individualized and highly selective IO (immune oncology) concept resulting in a well-tolerable cellular immune therapy
- APN401 preferentially reactivates tumor-specific, cytotoxic immune cells of the patient immune system through ex vivo blockade of Cbl-b
- APN401 follows a transient Cbl-b silencing concept attributing APN401 a superior safety profile in a multiple treatment setup
- APN401 can be manufactured within hours allowing blood cell isolation, immune cell processing and patient infusion in an ambulatory setting
- APN401 meets high-end ATMP (advanced therapy medicinal product) and safety standards due to closed, automated and GMP compliant cell manufacturing and release testing
Which technology platform is used for APN401 ?
- The technology platform used for APN401 is a closed, modular, automated cell manufacturing system using a highly effective small interfering siRNA to silence Cbl-b in patient blood cells.
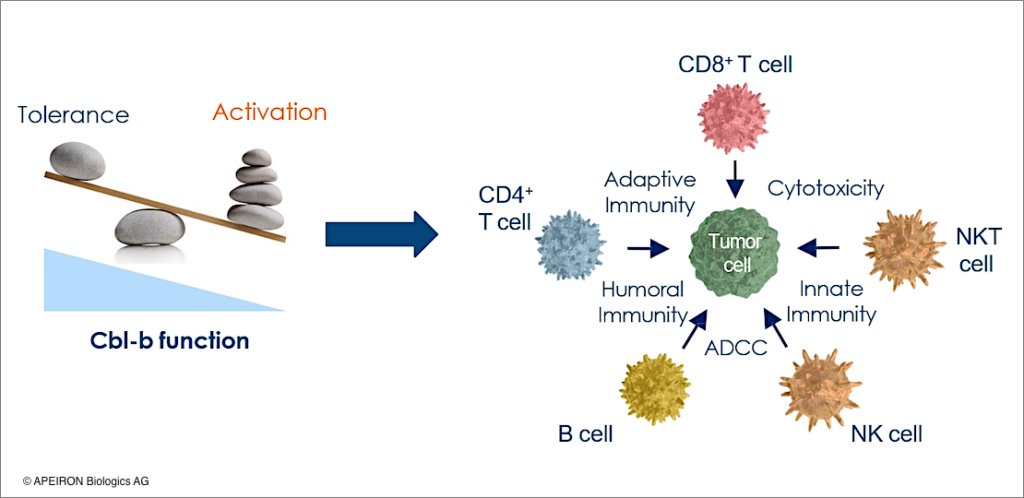
- Cbl-b in its role as a negative regulator of innate and adaptive immunity represents a dominant factor in tumor-triggered immune evasion.
- Due to immune inhibitory functions in different immune cell subsets Cbl-b is considered as a master immune checkpoint, hence, representing an ideal IO target. As a consequence, blocking Cbl-b function upon silencing the Cbl-b gene in a variety of immune cells may lead to the reactivation of immune processes that help eradicating tumor cells (Figure 1).
- Along these lines, Cbl-b deficiency strengthens T and NK cell cytotoxicity as well as antibody-dependent cell mediated cytotoxicity (ADCC) against tumor cells.
Where do we stand in the development of APN401 ?
- APN401 was tested well-tolerated in a Phase 1 study using an open, manual manufacturing system.
- Currently, we are preparing a Phase 1b multiple ascending dose study using our closed, automated manufacturing system.
We are planning to start recruitment in Q2 2021. - In this upcoming study, we want to evaluate the clinical applicability of APN401 by showing:
- feasibility of processing an effective cell dosage,
- determining the safety profile at an effective cell dosage, which is characterized by
- increased anti-tumor immune reactivity.
- Finally, we will close the P1b trial by evaluating the clinical response at an immunologically effective cell dosage by treating selected cancer patients with colon, lung as well as head and neck carcinoma.
Please read more about APEIRON Biologics here.
Here you can see our pipeline.
Follow up on our latest development in our press section.
We are committed to act and imagine a world where we can prevent people dying from cancer.
Our Cell Therapy Team at APEIRON Biologics AG

